Association of the Neutrophil-to-Lymphocyte Ratio (NLR) with Survival in Locally Advanced
and Metastatic Cervical Cancer: A Longitudinal, Retrospective Study
Article / Artículo
https://doi.org/10.33821/778
Date received: 19/1/2025
Date accepted: 30/3/2025
Date: 30/4/2025
1. Introduction
Cervical cancer (CC) is the second most common type of gynecological cancer worldwide,
with approximately 528,000 new cases and 266,000 deaths annually [1,2]. Although its incidence and mortality have declined in developed countries, it remains
a leading cause of death in developing regions. It accounts for 84% of cases, with
high rates reported in Africa, Central America, Latin America, and the Caribbean.
The average age at diagnosis is 48 years, with lower prevalence among women under
20 and over 85 years old [1,2,3]. In Ecuador, it ranks second in incidence and first in mortality, according to data
from SOLCA-Quito and GLOBOCAN [4,5,6].
Key prognostic factors include tumor stage (FIGO, International Federation of Gynecology
and Obstetrics), lymph node status, tumor size (TNM), and histologic grade. However,
clinical staging has limitations, especially in advanced stages, highlighting the
need for additional methods such as the neutrophil-to-lymphocyte ratio (NLR) [7]. Inflammation plays a crucial role in tumorigenesis, and NLR has been proposed as
a useful marker to predict prognosis in solid tumors [8,9,10]. Several studies have shown that chemotherapy can normalize NLR, which is associated
with better treatment responses [11-14].
Human papillomavirus (HPV) is detected in 99.7% of cases, with its persistence being
the main factor in cancer development [15-17]. In locally advanced stages (IB2-IVA), recurrence rates reach 50-70%, and metastatic
disease occurs in 15-61% of cases [18,19,20]. The standard treatment involves platinum-based chemotherapy combined with radiotherapy.
Risk stratification using Moore criteria is essential to individualize treatment [18,19,21-25]. Mizunuma [26] demonstrated that a high pretreatment NLR is associated with poorer overall and
progression-free survival [27]. This underscores the importance of this marker in optimizing resource use and improving
patients' quality of life.
2. Materials and Methods
This study was conducted as an observational, descriptive, historical cohort study
with a longitudinal, retrospective, and analytical approach to survival. It was carried
out at the SOLCA Oncology Hospital in Quito, and included patients diagnosed with
locally advanced cervical cancer (stages IB2 to IVA) and metastatic disease (stage
IVB), treated between January 2010 and January 2018. Inclusion criteria encompassed
the availability of complete blood count data, measurable disease on imaging, histologic
subtype determination, and having received cancer treatment in accordance with clinical
stage.
Prior to its implementation, the study was approved by the Ethics and Human Research
Committee (CEISH) of SOLCA Quito. Data were anonymized through a de-identification
process that irreversibly removed any information that could identify individuals,
using coded identifiers to mask patient identity. Given the retrospective nature of
the study, no interventions were performed that could affect patient care, and informed
consent was therefore not required.
Statistical analysis was conducted using IBM-SPSS software, which facilitated the
application of various statistical tests to examine the relationship between the neutrophil-to-lymphocyte
ratio (NLR) and survival in patients with cervical cancer. First, descriptive analyses
were performed to obtain measures of central tendency (mean, median) and dispersion
(standard deviation) for quantitative variables, as well as frequency distributions
for categorical variables.
Overall survival (OS) was defined as the time from the initiation of cancer treatment
to the date of death from any cause or the last follow-up visit in censored patients.
Recurrence-free survival (RFS) was defined as the interval from treatment initiation
to the clinical or radiological detection of the first tumor recurrence. Both variables
were analyzed using the Kaplan-Meier method to estimate cumulative survival probability
over time. OS and RFS curves were compared between risk groups based on NLR (<2.5
vs. ≥2.5). The log-rank test was used to assess whether differences between groups
were statistically significant.
Additionally, a Cox proportional hazards regression model was applied for multivariate
analysis, enabling the identification of independent prognostic factors for survival,
adjusted for potential confounders. This model calculated hazard ratios (HRs) and
their confidence intervals, providing an estimate of the relative risk of survival
events. The proportional hazards assumption was verified using the Schoenfeld residuals
test. All p-values were two-sided, and a p-value <0.05 was considered statistically
significant.
2.1 Inclusion criteria
-
Age: between 18 and 80 years.
-
Patients diagnosed with cervical cancer, FIGO 2009 clinical stages IB2 to IVB.
-
Patients treated at the SOLCA Oncology Hospital - Quito, from 2010 to 2018, with concurrent
chemoradiotherapy or chemotherapy alone.
-
Measurable oncologic disease according to RECIST 1.0 criteria.
2.2 Exclusion criteria
-
Non-cancer-related conditions that could alter the NLR, such as pre-existing cardiovascular
disease (e.g., hypertension), diabetes mellitus, acute or chronic kidney disease,
or infection-related processes (e.g., sepsis, superimposed infection prior to treatment
initiation).
-
Patients who underwent non-oncologic surgery as treatment.
-
Use of alternative therapies or treatment discontinuation.
-
Diagnosis of a second primary malignancy.
-
Patients who received radiotherapy alone as treatment.
A total of 3,080 patients were initially evaluated. After applying the exclusion criteria,
2,408 were excluded, including patients with comorbidities: 542 (history of cardiovascular
disease, acute or chronic kidney disease, metabolic diseases such as diabetes mellitus,
and autoimmune disorders), 1,366 (treated with radiotherapy alone), and 500 (treatment
discontinuation) (Fig. 1).
All patients had their white blood cell counts assessed before treatment initiation,
at treatment completion, and at the time of recurrence. Differential white blood cell
counts (neutrophils, lymphocytes, among others) were measured using automated hematology
analyzers.
Treatment response was evaluated after the third cycle in patients with metastatic
disease, and after the fifth week in patients with locally advanced disease, using
full body computed tomography and assessed according to RECIST 1.0 criteria, which
were in use at the institution at the time of analysis.
Figure 1
Patient selection process
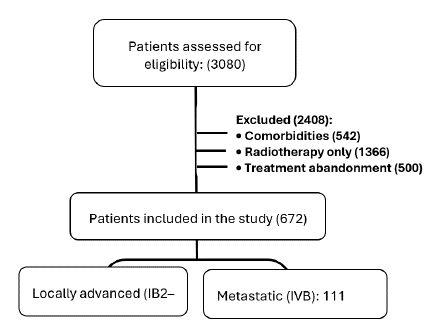
2.3 Results
A total of 672 patients met the inclusion criteria, representing 21.81% of the initial
cohort. The final study population was stratified into two groups according to clinical
stage: 561 patients (IB2-IVA) with locally advanced disease, and 111 patients (IVB)
with metastatic disease.
The mean age was 48.7 years (SD ±11.4). The mean white blood cell count was 9,049/μL,
with a mean lymphocyte count of 2,453/μL and neutrophil count of 5,438/μL. The mean
neutrophil-to-lymphocyte ratio (NLR) was 2.39 (SD ±1.43). Regarding histopathological
subtypes, squamous cell carcinoma predominated (85.6%), followed by adenocarcinoma
(10%) and adenosquamous carcinoma (4.5%).
Among the 672 patients analyzed, 229 (34.1%) had a high-risk NLR, while 443 (65.9%)
had a low-risk NLR. Based on Moore criteria, applied to patients with disease progression
and metastasis, two risk groups were identified: an intermediate-to-high-risk group
comprising 268 patients (39.9%), and a low-risk group with 34 patients (5.1%) (Table 1).
We conducted a contingency analysis of NLR in relation to vital status (alive, deceased)
and disease status (recurrence, no recurrence), using a cutoff value of 2.5 based
on previous literature and further supported by the mean NLR observed in our patient
population (2.39 ± 1.43). Patients with a low-risk NLR exhibited a mortality rate
of 32.7% and a recurrence rate of 32.4%. In comparison, those with a high-risk NLR
showed a mortality rate of 35.8% and a recurrence rate of 54.9%. These differences
were statistically significant (Table 2).
Table 1
Descriptives of the study population
|
Variables
|
Frequency (%)
|
|
Clinical stage (FIGO)
|
|
|
IIB
|
246 (36,6)
|
|
IIIA
|
1 (0,1)
|
|
IIIB
|
303 (45,1)
|
|
IVA
|
11 (1,6)
|
|
IVB
|
111 (16,5)
|
|
Leukocytosis
|
|
> 13000
|
55 (8,2)
|
|
≤ 13000
|
617 (91,8)
|
|
INL risk
|
|
|
High
|
229 (34,1)
|
|
Low
|
443 (65,9)
|
|
Moore criteria (risk)
|
|
Lowo
|
34 (5,1)
|
|
Intermediate-high
|
268 (39,9)
|
|
Vital status
|
|
Alive
|
402 (59,8)
|
|
Dead
|
270 (40,2)
|
Table 2
Analysis of recurrence and mortality (Chi-square)
|
Variables
|
Recurrence
|
Mortality
|
p-value
|
|
(%)
|
(%)
|
|
|
Neutrophil-to-Lymphocyte
|
High Risk ≥ 2.5
|
125 (54,9)
|
129 (35,8)
|
0,000
|
|
Ratio (NLR)
|
Low Risk < 2.5
|
144 (32,7)
|
143 (32,4)
|
0,000
|
|
Moore Criteria
|
Intermediate and High Risk
|
265 (98,9)
|
249 (92,9)
|
0,000
|
|
Low Risk
|
4 (11,4)
|
5 (14,7)
|
0,000
|
|
Leukocytosis
|
>13000
|
33 (60)
|
33 (60)
|
0,002
|
|
≤ 13000
|
237 (38,4)
|
239 (38,7)
|
0,002
|
|
Neutrophils
|
> 7500
|
58 (54,7)
|
58 (54,7)
|
0,001
|
|
< 7500
|
212 (37,5)
|
214 (37,8)
|
0,001
|
|
Lymphocytopenia
|
> 1000
|
265 (39,9)
|
267 (40,2)
|
0,195
|
|
≤ 1000
|
5 (62)
|
5 (62,5)
|
0,202
|
An association analysis using Moore criteria revealed that women classified as intermediate-to-high
risk exhibited a recurrence rate of 98.9% and a mortality rate of 92.9% (p = 0.000).
Survival analysis comparing high- and low-risk NLR groups among patients classified
as intermediate-to-high versus low risk according to Moore criteria was conducted
using the Kaplan-Meier method, with statistical significance evaluated via the log-rank
test. The follow-up period extended up to 180 months. Regarding overall survival and
NLR, the median survival for patients with NLR ≥2.5 was 37 months, with a 95% confidence
interval (CI) of 26.3 to 47.6 (p < 0.05) (Figure 2).
Recurrence-free survival in relation to NLR showed a median of 30 months for patients
with NLR ≥ 2.5 (95% CI: 9.4-50.5; p < 0.05) (Figure 3).
Figure 2
Pre-treatment INL Overall Survival
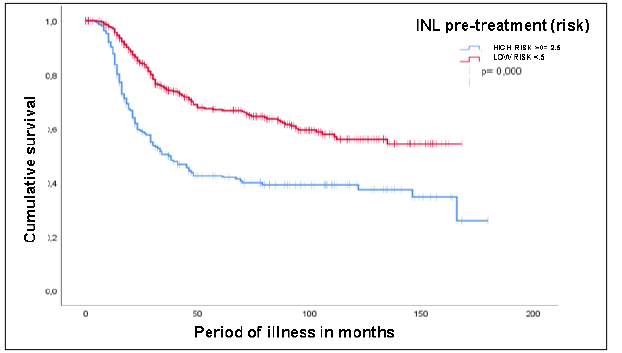
Source: Medical records from the SOLCA-Quito Cancer Hospital
Figure 3
Recurrence-free survival
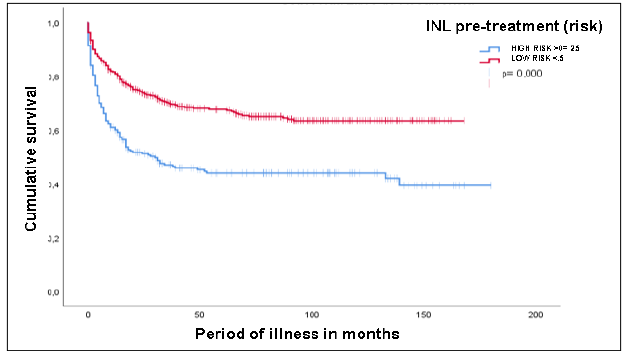
Source: Medical records from the SOLCA-Quito Cancer Hospital
The analysis of recurrence and mortality revealed significant associations with several
prognostic factors. A high-risk NLR (≥2.5) was strongly associated with increased
risk of disease recurrence (HR: 3.16, 95% CI: 2.47-4.05, p < 0.000) and mortality
(HR: 3.09, 95% CI: 2.42-3.94, p < 0.000). Moore criteria (intermediate and high risk)
showed a markedly higher risk, particularly for recurrence (HR: 10.66, 95% CI: 3.97-28.64,
p < 0.000) and mortality (HR: 5.39, 95% CI: 2.00-14.48, p = 0.001). Leukocytosis (>13,000)
and elevated neutrophil counts (>7,500) were also associated with increased risk in
both outcomes, with hazard ratios of 2.11 and 1.79, respectively (p < 0.000). Finally,
lymphocytopenia (<1,000) was significantly associated with both recurrence and mortality
risk (HR: 2.43, p < 0.000). These findings highlight the prognostic value of these
hematologic biomarkers as predictors of poor outcomes (Table 3).
Table 3
Hazard Ratio
|
|
Recurrence
|
Mortality
|
|
VARIABLES
|
HR
|
IC 95%
|
p-value
|
HR
|
IC 95%
|
p-value
|
|
High-risk NLR ≥ 2.5
|
3,16
|
2,47-4,05
|
0,000
|
3,09
|
2,42-3,94
|
0,000
|
|
Moore criteria (Intermediate and High)
|
10,66
|
3,97-28,64
|
0,000
|
5,39
|
2,00-14,48
|
0,001
|
|
Leukocytosis (>13,000)
|
2,14
|
1,48-3,08
|
0,000
|
2,11
|
1,48-3,08
|
0,000
|
|
Neutrophils (>7,500)
|
1,79
|
1,34-2,40
|
0,000
|
1,79
|
1,34-2,40
|
0,000
|
|
Lymphocytopenia (≤ 1,000)
|
2,43
|
1,00-5,90
|
0,000
|
2,43
|
1,00-5,90
|
0,000
|
3. Discussion
This study assessed the prognostic value of hematological and inflammatory markers
in patients with cervical cancer (CC). The main findings indicate that a pre-treatment
neutrophil-to-lymphocyte ratio (NLR) ≥ 2.5 is associated with a higher risk of early
recurrence and reduced overall survival (OS), with medians of 30 and 37 months, respectively,
and a significant hazard ratio (HR) for recurrence (3.16) and OS (3.09).
These results are consistent with those reported by Mizunuma et al. [13], who identified NLR ≥ 2.5 as a prognostic factor for both OS and progression-free
survival (PFS), although with slightly longer periods (PFS of 35.9 months and OS of
37.7 months). Wang et al. [27] also reported differences in OS for NLR ≥ 2.5 values, while Onal et al. [28] confirmed the relevance of NLR as a predictor for both OS and PFS.
Other markers, such as leukocytosis and neutrophilia, were also associated with worse
prognosis [29]. In this study, leukocytosis doubled the risk of recurrence and mortality (HRs of
2.14 and 2.11), in line with Cho et al. [30] and Mabuchi et al. [31], who reported significantly reduced OS in patients with tumor-associated leukocytosis.
Neutrophilia was associated with earlier recurrence (HR 1.79) and shorter OS (38 months,
HR 2.43), similar to findings by Matsumoto et al. [32] in granulocyte-colony stimulating factor (G-CSF)-producing tumors.
Regarding lymphocytopenia, although it did not show statistical significance on its
own, it was associated with early recurrence and reduced OS when combined with other
factors. This aligns with findings from studies such as that of Clark et al. [33] in pancreatic cancer.
The validity of the Moore risk model was also highlighted. This model stratifies patients
into low, intermediate-, and high-risk categories, showing a direct correlation between
increased risk and worse prognosis. These findings are supported by the study by Tewari
[34], in which patients in the intermediate- and high-risk groups derived significant
OS benefit from treatment with bevacizumab.
Systemic inflammatory response is characterized by an imbalance in white blood cell
populations, mainly neutrophilia with relative lymphocytopenia [35-37]. Although isolated counts of lymphocytes or neutrophils may hold prognostic value
in CC [38,39], their variability limits their standalone utility; therefore, it is recommended
to combine them with other parameters to enhance their accuracy as prognostic indicators.
Finally, it is emphasized that elevated NLR is associated with impaired antitumor
immune response. Lymphocytes play a crucial role in immune surveillance, while tumor-associated
neutrophils promote tumor progression by inducing an inflammatory and immunosuppressive
microenvironment. Moreover, chronic systemic inflammation mediated by cytokines such
as IL-6, TNF-α, and G-CSF is believed to contribute to CC progression [40,41].
The results of this study should be validated in larger, multicenter prospective studies.
Since the study was conducted in a single institution, there is a potential risk of
selection bias, which may limit the generalizability of the findings to other populations.
Including multiple centers could yield a more diverse and representative sample and
improve the external validity of the results.
4. Conclusions
This study demonstrated that a low-risk NLR (< 2.5) is associated with higher survival
rates and fewer cases of recurrence across all stages of cervical cancer. This evaluation
enables a more precise stratification of patients, facilitating the personalization
of treatment and optimizing healthcare costs related to this pathology. In contrast,
an NLR ≥ 2.5 was associated with more aggressive tumor behavior, as evidenced by a
higher probability of early recurrence and increased mortality.
Moreover, the importance of applying Moore criteria as a routine practice for prognostic
evaluation in patients with recurrent and metastatic cervical cancer was highlighted.
The study also revealed that leukocytosis has significant prognostic value in terms
of survival and recurrence. Since the presence of infections was an exclusion criterion,
it is concluded that treatment delays due to these conditions increase complications
and accelerate disease progression.
1. Introducción
El cáncer de cérvix uterino (CACU) es el segundo tipo más común de cáncer ginecológico
a nivel mundial, con aproximadamente 528 000 nuevos casos y 266 000 muertes anuales
[1,2]. Aunque su incidencia y mortalidad han disminuido en países desarrollados, sigue
siendo una causa principal de muerte en regiones en desarrollo. Representa el 84 %
de los casos, con tasas elevadas en África, América Central, Latinoamérica y el Caribe.
La edad media de diagnóstico es de 48 años, con una menor prevalencia en mujeres menores
de 20 años y mayores de 85 años [1-3]. En Ecuador, ocupa el segundo lugar en incidencia y el primero en mortalidad, según
datos de SOLCA-Quito y GLOBOCAN [4-6].
Los factores pronósticos clave incluyen el estadio tumoral, según la Federación Internacional
de Ginecología y Obstetricia (FIGO), el estado de ganglios linfáticos, el tamaño tumoral
y el grado histológico. Sin embargo, la estadificación clínica tiene limitaciones,
sobre todo en etapas avanzadas, lo que muestra la necesidad de usar métodos adicionales,
como el índice neutrófilo/linfocitos (INL) [7]. La inflamación juega un papel crucial en la tumorigénesis, y el INL se ha propuesto
como un marcador útil para predecir la prognosis en tumores sólidos [8-10]. Diversos estudios han demostrado que la quimioterapia puede normalizar el INL,
y se asocia con mejores respuestas al tratamiento [11-14].
El virus del papiloma humano (VPH) es detectado en el 99,7 % de los casos; su persistencia
es el principal factor para el desarrollo del cáncer [15-17]. En etapas localmente avanzadas (IB2-IVA), las tasas de recurrencia alcanzan el
50-70 %, y la enfermedad metastásica se presenta en el 15-61 % de los casos [18-20]. El tratamiento estándar combina quimioterapia basada en platino y radioterapia.
La estratificación del riesgo mediante los criterios de Moore es esencial para individualizar
el tratamiento [18,19,21-25]. Mizunuma [26] mostró que un INL alto en el pretratamiento se relaciona con una menor supervivencia
general y libre de progresión [27]. Esto enfatiza la importancia de este marcador para usar mejor los recursos y mejorar
la calidad de vida de las pacientes.
2. Materiales y métodos
La investigación fue observacional, descriptiva de cohortes históricas, con un enfoque
longitudinal, retrospectivo y analítico de sobrevida. Se realizó en el Hospital Oncológico
SOLCA, núcleo Quito, e incluyó a pacientes diagnosticadas con CACU localmente avanzado
(etapas IB2 a IVA) y metastásico (etapa IVB), tratadas entre enero del 2010 y enero
del 2018. Los criterios de inclusión abarcaron la disponibilidad de biometría hemática,
enfermedad medible por imagen, determinación del tipo histológico y haber recibido
tratamiento oncológico acorde a su etapa clínica.
Previo a su ejecución, el estudio obtuvo la aprobación del Comité de Ética e Investigación
en Seres Humanos (CEISH) de SOLCA, núcleo Quito. Los datos se anonimizaron mediante
un proceso de desidentificación que elimina de forma irreversible toda información
que pudiera identificar a los individuos y utiliza códigos para enmascarar su identidad.
Dado el diseño retrospectivo del estudio, no se realizó ninguna intervención que pudiera
afectar a las pacientes, por lo que no fue necesario aplicar el consentimiento informado.
El análisis estadístico del estudio se llevó a cabo con el programa IBM-SPSS, lo que
facilitó la aplicación de diferentes pruebas estadísticas para examinar la relación
entre el INL y la supervivencia en pacientes con CACU. En primer lugar, se llevaron
a cabo análisis descriptivos para obtener medidas de tendencia central (media, mediana)
y dispersión (desviación estándar [DE]) de las variables cuantitativas, así como distribuciones
de frecuencia para las variables categóricas.
La supervivencia global (SG) se definió como el tiempo transcurrido desde la fecha
de inicio del tratamiento oncológico hasta la fecha de muerte por cualquier causa
o la última visita de seguimiento en caso de pacientes censurados. La supervivencia
libre de recurrencia (SLR) se definió como el intervalo entre el inicio del tratamiento
y la detección clínica o radiológica de la primera recurrencia tumoral. Ambas variables
se analizaron utilizando el método de Kaplan-Meier, que estima la probabilidad acumulada
de supervivencia en función del tiempo. Se compararon las curvas de SG y SLR entre
grupos de riesgo con base en el INL (INL < 2,5 vs. INL ≥ 2,5). Se utilizó la prueba
de logrank para analizar si las diferencias entre los grupos eran estadísticamente
significativas.
Además, se empleó el modelo de regresión de Cox para llevar a cabo un análisis multivariado,
lo que permitió identificar factores pronósticos independientes de supervivencia,
ajustados por variables potencialmente confundentes. Este modelo calculó los hazard
ratio (HR) y sus intervalos de confianza, y proporcionó una estimación del riesgo
relativo de eventos de supervivencia. Se verificó la proporcionalidad de los riesgos
utilizando la prueba de Schoenfeld. Los valores de p fueron bilaterales y se consideró
p < 0,05 como significativo para todos los análisis.
2.1 Criterios de inclusión
-
Edad entre 18 y 80 años.
-
Pacientes con diagnóstico de CACU estadio clínico IB2 al IVB, de acuerdo con el sistema
de estadificación FIGO 2009.
-
Pacientes que recibieron tratamiento en el Hospital Oncológico SOLCA, núcleo Quito,
del 2010 al 2018, concurrencia y quimioterapia sola.
-
Enfermedad oncológica medible según criterios RECIST 1.0.
2.2 Criterios de exclusión
Condiciones no relacionadas con cáncer (comorbilidades), en las que se puede alterar
el INL, como enfermedad cardiovascular preexistente (hipertensión arterial), diabetes
mellitus, enfermedad renal aguda, crónica o proceso infeccioso relacionado (sepsis,
infección sobreañadida previa al inicio de tratamiento).
-
Pacientes sometidas a cirugía no oncológica, como tratamiento.
-
Terapia alternativa y abandono de tratamiento.
-
Diagnóstico de doble primario.
-
Pacientes que hubieran recibido solo radioterapia como tratamiento.
Se evaluó a 3080 pacientes inicialmente y, luego, por criterios de exclusión, se retiró
del estudio a 2408 pacientes, entre ellas portadoras de comorbilidades: 542 con antecedentes
personales de enfermedades cardiovasculares, enfermedad renal aguda y crónica, enfermedades
metabólicas como la diabetes mellitus, enfermedades autoinmunes; 1366 pacientes tratadas
solo con radioterapia, y 500 por abandono del tratamiento (Figura 1).
Todas las pacientes tuvieron su recuento de glóbulos blancos antes de iniciar tratamiento,
al final y a la recurrencia. Los recuentos de leucocitos diferenciales de neutrófilos,
linfocitos, entre otros, se midieron utilizando contadores de células sanguíneas automatizados.
La evaluación de respuesta al tratamiento se realizó luego del tercer ciclo en las
pacientes con enfermedad metastásica y luego de la quinta semana en las pacientes
con enfermedad localmente avanzada, con tomografía corporal y evaluadas según criterios
RECIST 1.0, que para la fecha del análisis se aplicaba en la institución.
Figura 1
Proceso de selección de pacientes
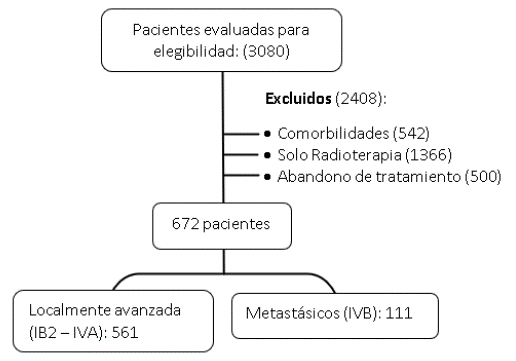
2.3 Resultados
Las pacientes que cumplieron los criterios de inclusión fueron 672 (21,81 %). En el
conjunto final analizado se identificaron dos grupos según el estadio clínico: el
localmente avanzado con 561 pacientes (IB2-IVA) y el metastásico con 111 pacientes
(IVB).
La edad promedio fue de 48,7 años con DE 11,4; la media del conteo de leucocitos fue
de 9049; el de linfocitos, de 2453, y el de neutrófilos, de 5438. En el INL, la media
fue de 2,39 con DE 1,43. En cuanto al tipo histopatológico, predominó el de células
escamosas con 85,6 %, seguido del adenocarcinoma en 10 % y el adenoescamoso con un
4,5 %.
En relación con el INL de las 672 pacientes analizadas, 229 (34,1 %) tenían un INL
de alto riesgo y 443 (65,9 %) tenían un INL de bajo riesgo. En los resultados de los
criterios de Moore, aplicados a las pacientes con progresión de enfermedad y metástasis,
se identificaron dos grupos: uno de riesgo intermedio-alto, que tuvo 268 (39,9%) pacientes,
y otro de bajo riesgo, con 34 (5,1 %) pacientes (Tabla 1).
Se llevó a cabo un análisis de contingencia para INL en relación con el estado vital
(vivo, muerto) y el estado de la enfermedad (recurrencia, no recurrencia), con un
corte del INL de 2,5 por reporte en la literatura y soportado adicionalmente por la
media obtenida en las pacientes (2,39 ± 1,43). Se observó que las pacientes con INL
de bajo riesgo tienen una mortalidad del 32,7 % y una recurrencia del 32,4 %. En comparación,
las mujeres con INL de alto riesgo presentan una mortalidad del 35,8 % y una recurrencia
del 54,9 %. Estos resultados son estadísticamente significativos (Tabla 2).
Tabla 1
Descriptivos de la población de estudio
|
Variables
|
Frecuencia (%)
|
|
Estadio clínico (FIGO)
|
|
IIB
|
246 (36,6)
|
|
IIIA
|
1 (0,1)
|
|
IIIB
|
303 (45,1)
|
|
IVA
|
11 (1,6)
|
|
IVB
|
111 (16,5)
|
|
Leucocitosis
|
|
> 13000
|
55 (8,2)
|
|
≤ 13000
|
617 (91,8)
|
|
INL riesgo |
|
|
Alto
|
229 (34,1)
|
|
Bajo
|
443 (65,9)
|
|
Criterios de Moore (riesgo)
|
|
Bajo
|
34 (5,1)
|
|
Intermedio-alto
|
268 (39,9)
|
|
Estado vital
|
|
|
Viva
|
402 (59,8)
|
|
Muerta
|
270 (40,2)
|
Tabla 2
Análisis de recurrencia y mortalidad (ji al cuadrado)
|
Variables
|
Recurrencia
|
Mortalidad
|
Valor p
|
|
Recuento (%)
|
Recuento (%)
|
|
Índice neutrófilos/ linfocitos (INL)
|
Alto riesgo ≥ 2,5
|
125 (54,9)
|
129 (35,8)
|
0,000
|
|
Bajo Riesgo < 2,5
|
144 (32,7)
|
143 (32,4)
|
0,000
|
|
Criterios de Moore
|
Intermedio y Alto Riesgo
|
265 (98,9)
|
249 (92,9)
|
0,000
|
|
Bajo Riesgo
|
4 (11,4)
|
5 (14,7)
|
0,000
|
|
Leucocitosis
|
> 13000
|
33 (60)
|
33 (60)
|
0,002
|
|
≤ 13000
|
237 (38,4)
|
239 (38,7)
|
0,002
|
|
Neutrófilos
|
> 7500
|
58 (54,7)
|
58 (54,7)
|
0,001
|
|
≤ 7500
|
212 (37,5)
|
214 (37,8)
|
0,001
|
|
Linfocitopenia
|
> 1000
|
265 (39,9)
|
267 (40,2)
|
0,195
|
|
≤ 1000
|
5 (62)
|
5 (62,5)
|
0,202
|
Al realizar el análisis de asociación para los criterios de Moore, se encontró que
las mujeres de riesgo intermedio y alto tienen una recurrencia del 98,9 % y mortalidad
del 92,9 % con p 0,0001.
El análisis de supervivencia en los grupos de INL de alto riesgo y de bajo riesgo
en las pacientes que cumplían criterios de riesgo intermedio-alto en comparación con
bajo riesgo de los criterios de Moore se realizó mediante la técnica de Kaplan-Meier
y evaluación de significancia estadística con prueba de logrank, con un periodo de
seguimiento de 180 meses. En SG, en relación con el INL, la mediana de supervivencia
para las pacientes con INL ≥ 2,5 fue de 37 meses, con intervalo de confianza (IC)
del 95 % de 26,3 a 47,6 con p < 0,05 (Figura 2).
Respecto a la SLR en relación con el INL, se encontró una mediana para las pacientes
con INL ≥ 2,5 de 30 meses (IC 95 % de 9,4-50,5; p < 0,05) (Figura 3).
Figura 2
Supervivencia global INL pretratamiento
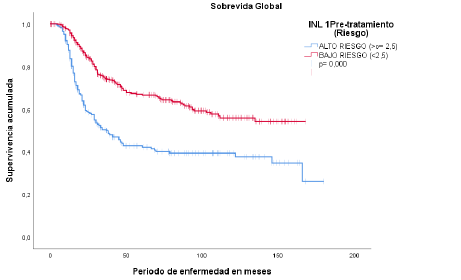
Fuente: historias clínicas del Hospital Oncológico SOLCA, núcleo Quito.
Figura 3
Supervivencia libre de recurrencia
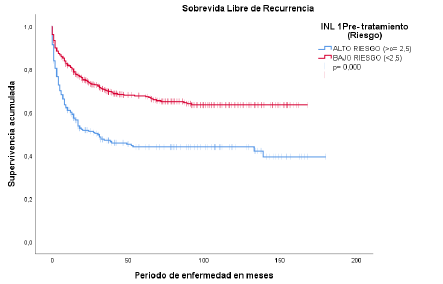
Fuente: historias clínicas del Hospital Oncológico SOLCA, núcleo Quito.
El análisis de recurrencia y mortalidad reveló asociaciones significativas con varios
factores pronósticos. Un INL de alto riesgo (≥ 2,5) se asoció con un aumento notable
en el riesgo de que la enfermedad regrese (HR: 3,16, IC 95 %: 2,47-4,05, p < 0,000)
y en la mortalidad (HR: 3,09, IC 95 %: 2,42-3,94, p < 0,000). Los criterios de Moore
(riesgo intermedio y alto) mostraron un riesgo mucho mayor, sobre todo en cuanto a
recurrencia (HR: 10,66, IC 95 %: 3,97-28,64, p < 0,000) y mortalidad (HR: 5,39, IC
95 %: 2,00-14,48, p = 0,001). La leucocitosis (> 13000) y el aumento de neutrófilos
(> 7500) se relacionaron con un mayor riesgo en ambas mediciones, con un HR de 2,11
y 1,79, respectivamente (p < 0,000). Finalmente, la linfocitopenia (< 1000) mostró
una relación significativa con el riesgo de recurrencia y mortalidad (HR: 2,43, p
< 0,000). Estos hallazgos subrayan la importancia de estos biomarcadores como predictores
de mal pronóstico (Tabla 3).
Tabla 3
Hazard ratios (HR)
|
|
Recurrencia
|
Mortalidad
|
|
VARIABLES
|
HR
|
IC 95 %
|
Valor p
|
HR
|
IC 95 %
|
Valor p
|
|
INL Alto riesgo ≥2,5
|
3,16
|
2,47-4,05
|
0,000
|
3,09
|
2,42-3,94
|
0,000
|
|
Criterios de Moore-riesgo (intermedio y alto)
|
10,66
|
,97-28,64
|
0,000
|
5,39
|
2,00-14,48
|
0,001
|
|
Leucocitosis (> 13000)
|
2,14
|
1,48-3,08
|
0,000
|
2,11
|
1,48-3,08
|
0,000
|
|
Neutrófilos (> 7500)
|
1,79
|
1,34-2,40
|
0,000
|
1,79
|
1,34-2,40
|
0,000
|
|
Linfocitopenia (< 1000)
|
2,43
|
1,00-5,90
|
0,000
|
2,43
|
1,00-5,90
|
0,000
|
3. Discusión
El estudio evaluó el valor pronóstico de marcadores hematológicos e inflamatorios
en pacientes con CACU. Los principales hallazgos indican que un INL ≥ 2,5 previo al
tratamiento se asocia con mayor riesgo de recurrencia temprana y menor SG, con medianas
de 30 y 37 meses, respectivamente, y un HR significativo para recurrencia (3,16) y
SG (3,09).
Estos resultados son comparables con los reportados por Mizunuma et al. [13], quienes hallaron un INL ≥ 2,5 como un factor pronóstico para SG y SLR, aunque con
periodos ligeramente más prolongados (SLR de 35,9 meses y SG de 37,7 meses). Wang
et al. [27] también identificaron diferencias en SG para valores de INL ≥ 2,5, mientras que
Onal et al. [28] confirmaron la relevancia del INL como predictor tanto para SG como para SLR.
Otros marcadores como la leucocitosis y la neutrofilia también se relacionaron con
un peor pronóstico [29]. En este estudio, la leucocitosis duplicó el riesgo de recurrencia y mortalidad
(HR de 2,14 y 2,11), lo que coincide con Cho et al. [30] y Mabuchi et al. [31], quienes demostraron una SG significativamente menor en pacientes con leucocitosis
asociada al tumor. La neutrofilia se asoció con recurrencia más temprana (HR 1,79)
y SG más corta (38 meses, HR 2,43), similar a lo reportado por Matsumoto et al. [32] en tumores productores de G-CSF.
Respecto a la linfocitopenia, aunque no mostró significancia estadística por sí sola,
se relacionó con recurrencia temprana y menor SG al combinarse con otros factores,
lo cual se alinea con estudios como el de Clark et al. [33] en cáncer pancreático.
Se destacó la validez del modelo de riesgo de Moore, que clasifica a los pacientes
en categorías de bajo, intermedio y alto riesgo, y muestra correlación directa entre
mayor riesgo y peor pronóstico. Estos hallazgos fueron respaldados por el estudio
de Tewari [34], en el que los grupos de riesgo intermedio y alto obtuvieron un beneficio notable
en SG al recibir bevacizumab.
La respuesta inflamatoria sistémica se caracteriza por un desequilibrio en las poblaciones
de glóbulos blancos, principalmente por neutrofilia con linfocitopenia relativa [35 - 37]. Aunque el recuento aislado de linfocitos o neutrófilos puede tener valor pronóstico
en CACU [38,39], su variabilidad limita su utilidad individual; por ello, se recomienda combinarlos
con otros parámetros para mejorar su precisión como indicadores pronósticos.
Finalmente, se enfatiza que el INL elevado se relaciona con una respuesta inmunológica
antitumoral comprometida. Los linfocitos desempeñan un papel crucial en la vigilancia
inmune, mientras que los neutrófilos asociados a tumores favorecen la progresión tumoral
al inducir un microambiente inflamatorio e inmunosupresor. Además, se sugiere que
la inflamación sistémica crónica, mediada por citoquinas como IL-6, TNF-α y G-CSF,
contribuye a la progresión del CACU [40,41].
Los resultados del presente estudio deben ser validados en estudios prospectivos más
amplios y multicéntricos, pues, al haberse realizado en una sola institución, existe
el riesgo de sesgo de selección, lo que podría limitar la generalización de los hallazgos
a otras poblaciones. La inclusión de múltiples centros podría proporcionar una muestra
más diversa y representativa, y mejorar la validez externa de los resultados.
4. Conclusiones
El estudio mostró que un INL de bajo riesgo (< 2,5) está relacionado con una mayor
tasa de supervivencia y menos casos de recurrencia en todas las etapas del CACU. Esta
evaluación permite una estratificación más precisa de las pacientes, lo que facilita
la personalización del tratamiento y optimiza los costos sanitarios relacionados con
esta patología. Por el contrario, un INL ≥ 2,5 se asoció con un comportamiento tumoral
más agresivo, que se nota en una mayor probabilidad de recurrencia temprana y un aumento
en la mortalidad.
Además, se destacó la importancia de aplicar los criterios de Moore como una práctica
habitual para la evaluación pronóstica en pacientes con CACU recurrente y metastásico.
El estudio también reveló que la leucocitosis posee un valor pronóstico relevante
en términos de supervivencia y recurrencia. Dado que la presencia de infecciones fue
un criterio de exclusión, se concluye que los retrasos en el tratamiento debido a
estas afecciones aumentan las complicaciones y aceleran la progresión de la enfermedad.