Gastric Schwannoma: A Case Report
Case Report / Caso Clínico
https://doi.org/10.33821/742
Date received: 04/5/2023
Date accepted: 23/2/2024
1. Clinical case
A 61-year-old female with a medical history of type 2 diabetes mellitus treated with
hypoglycemic agents. No family history of cancer. She presents dyspepsia with clinical
symptoms of postprandial distress syndrome, evolving for one year without alarm signs.
No abnormalities were found in her lower digestive tract and hepatobiliary system.
Physical examination: Good general condition. Abdomen: Soft, depressible, not painful,
no masses, no visceromegaly, and the rest of the physical examination showed no irregularities.
In the blood tests, no deviations were observed.
(Figure 1). Computed Tomography (CT) of the Abdomen and Pelvis, there is evidence of thickening
of the gastric antrum wall associated with an exophytic growth tumor, measuring 79
x 84 x 88 mm. Enlarged lymph nodes are observed in the hepatic hilar region, celiac
trunk, and peripheral areas.
Figure 1
Computed tomography (CT) abdomen-pelvis
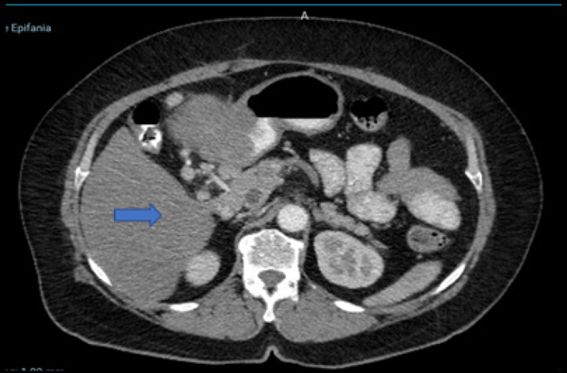
Source: Hospital SOLCA Núcleo Machala
Upper Endoscopy (EGD): At the level of the antrum, on the greater curvature, there
is a 4 cm subepithelial lesion covered with mucosa, suggesting a Gastrointestinal
Stromal Tumor (GISTs) based on its appearance (Figure 2).
Figure 2
Subepithelial lesion in the antrum, seen on upper endoscopy
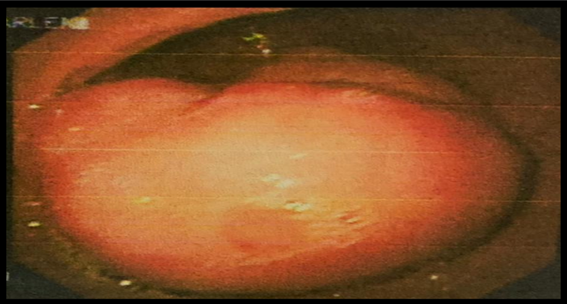
Source: Hospital SOLCA Núcleo Machala
An endoscopic ultrasound was performed: In the antrum, a hypoechoic lesion measuring
3 x 4 cm originates in the fourth layer (muscularis propria). It appeared heterogeneous
with anechoic areas inside and irregular contours. A 19 G ACQUIRE needle puncture
of the lesion is performed for pathological examination (Figure 3).
Figure 3
Subepithelial lesion in the antrum, seen on endoscopic ultrasound.
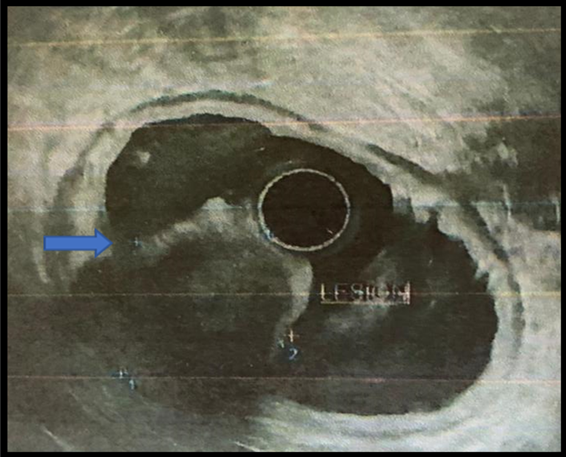
Source: Hospital SOLCA Núcleo Machala
The histopathological report describes infiltration of polymorphonuclear neutrophils
and mononuclear leukocytes, as well as a fragment of smooth muscle with a neoplastic
appearance formed by smooth, fusiform, bipolar muscle fibers with oval nuclei that
preserve the nucleocytoplasmic ratio. Immunohistochemistry (IHC) reveals S100 positivity
and desmid positivity, while Smooth Muscle Actin, CD34, and CD117 (c-Kit) are negative.
This establishes the diagnosis of Gastric Schwannoma (GS).
As definitive treatment, open surgery is performed: Subtotal gastrectomy. Anatomopathological
report: Gastric Schwannoma with perigastric lymph nodes showing reactive follicular
hyperplasia. In the 24-month follow-up, the patient remains asymptomatic regarding
digestive symptoms.
2. Discussion
Gastrointestinal subepithelial tumors are divided into 3 main groups: Neurogenic (schwannomas,
neurofibromas), myogenic (leiomyomas and leiomyosarcomas), and GISTs. Differential
diagnosis is crucial as they differ in prognosis [3].
Schwannomas are slow-growing benign mesenchymal tumors that originate from Schwann
cells of the Meissner and Auerbach plexuses. They are uncommon. Their most common
location in the gastrointestinal tract is the stomach (at the greater curvature and
antrum), followed by the colon and rectum [3,5].
Gastric Schwannomas are rare, having a frequency of 0.2% among all gastric tumors,
6.3% of mesenchymal gastric tumors, and 4% of benign gastric tumors. They are more
prevalent in women, with a male-to-female ratio of 1:3 and an average age at diagnosis
of 57 years [6,12].
The clinical presentation of Gastric Schwannomas can vary greatly. Most are asymptomatic
and are diagnosed incidentally. Symptomatic patients often present with abdominal
pain, followed by upper gastrointestinal bleeding. Less frequently, they may present
with a palpable abdominal mass (3%), loss of appetite (anorexia) (3%), or dyspepsia
(1.8%) [6].
Upper Endoscopy (EGD) and the biopsies obtained from it have low yield [4]. They often reveal sessile subepithelial tumors covered with mucosa of normal appearance
and exophytic growth.7 Endoscopic ultrasound identifies a hypoechoic lesion, either homogeneous or heterogeneous,
often with a marginal halo, located in the fourth layer and sometimes in the third
layer. Fine-Needle Aspiration (FNA) is the initial diagnostic method, providing a
diagnosis in 85.2% of cases. However, in instances where the obtained tissue is insufficient
or nonspecific, core needle biopsy may yield better results [3,8].
Another diagnostic tool used is contrast-enhanced CT, which shows a heterogeneously
hypervascular tumor that enhances with contrast, with areas of necrosis. However,
radiological findings are nonspecific and are often described as gastrointestinal
stromal tumor [7,13].
Histologically, Gastric Schwannomas are encapsulated tumors containing abundant spindle
cells with a prominent lymphoid aggregation characterized by Antoni A and Antoni B
areas. Shah AS et al [9]. demonstrated that the diagnosis can only be confirmed based on immunohistochemistry
(IHC), where GS shows positivity for S-100, vimentin, and glial fibrillary acidic
protein, and negativity for CD117 and Smooth Muscle Actin (SMA) [3,10,15].
Regarding treatment, surgery is the only curative treatment for GS, and the specific
type of procedure depends on the size and location of the lesion. Both conventional
and laparoscopic techniques have shown satisfactory results. Lymph node resection
is not necessary as SMA rarely presents lymphatic spread or malignant transformation.
Endoscopic options are not viable in most cases as the lesion usually arises from
the Auerbach plexus, and growths tend to involve the entire muscular layer. The recurrence
rate is very rare, so surveillance is not required [7, 10, 14].
3. Conclusions
Schwannomas are benign, slow-growing, mesenchymal tumors that originate in the Schwann
cells of the nerves of the Meiisner and Auebarch plexuses. They are uncommon in the
gastrointestinal tract. They should be taken into consideration in the differential
diagnosis of subepithelial lesions detected during endoscopy.
1. Caso clínico
Femenina de 61 años, antecedentes patológicos de diabetes mellitus tipo 2 en tratamiento
con hipoglucemiantes. Sin antecedentes familiares oncológicos.
Presenta cuadro clínico de dispepsia tipo distress postprandial, sin signos de alarmas,
de un año de evolución. Sin alteraciones en su tracto digestivo bajo o en la esfera
hepatobiliar. En el examen físico se identifica buen estado general. El abdomen se
palpa blando depresible, no doloroso, no tumoraciones, no visceromegalia. No se reflejan
particularidades en dicho examen. En la paraclínica sanguínea tampoco se hallan alteraciones.
En la tomografía computarizada de abdomen-pelvis (Figura 1) se evidencia, en antro gástrico, engrosamiento parietal asociado a tumoración con
crecimiento exofítico que mide 79 × 84 × 88 mm, y se aprecian ganglios incrementados
de tamaño en región hiliar hepática, tronco celíaco y periféricos.
Figura 1
Tomografía computarizada (TC) abdomen-pelvis
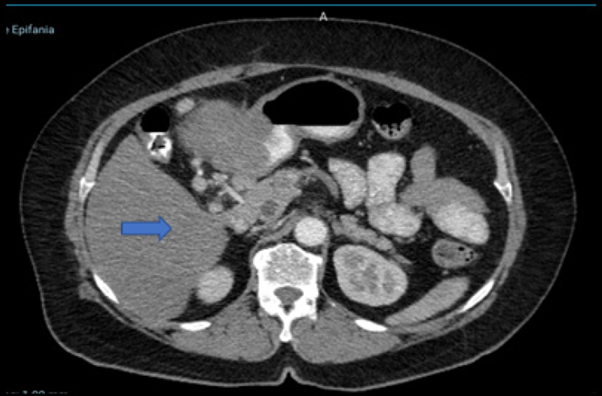
Fuente: Hospital SOLCA Núcleo Machala
En la endoscopia digestiva alta, a nivel de antro sobre curvatura, se refleja la mayor
lesión subepitelial de 4 cm, recubierta de mucosa de aspecto habitual sugestiva de
tumor del estroma gastrointestinal (GIST) (Figura 2).
Figura 2
Lesión subepitelial en antro vista por EDA
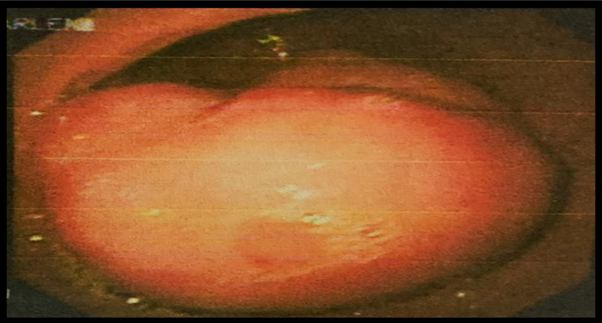
Fuente: Hospital SOLCA Núcleo Machala
Se realiza ecoendoscopia que evidencia: en antro, lesión hipoecoica de 3 × 4 cm, que
se origina en la cuarta capa (muscular propia), heterogénea, con áreas anecoicas en
su interior, contornos irregulares. Se realiza punción con aguja 19 G ACQUIRE de la
lesión para estudio de patología (Figura 3).
Figura 3
Lesión subepitelial en antro vista por ecoendoscopia
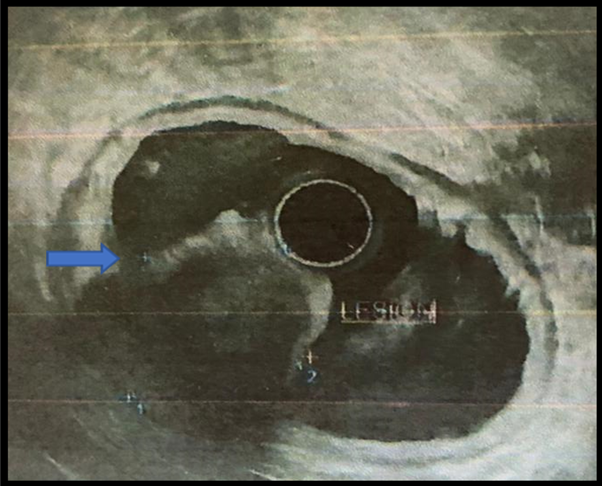
Fuente: Hospital SOLCA Núcleo Machala
Informe histopatológico describe infiltrado de leucocitos polimorfonucleares neutrófilos
y mononucleares, además de un fragmento de músculo liso de aspecto neoplásico, formado
por fibras musculares lisas, fusiformes, bipolares de núcleos ovalados que preservan
la relación núcleo-citoplasma. Se realiza inmunohistoquímica (IHQ): S100 positivo
y desmina, actina músculo específico, CD 34 Y CD 117 (c-Kit) negativos; estableciendo
diagnóstico de schwannoma gástrico (SG).
Como tratamiento definitivo, se realiza cirugía por vía abierta: gastrectomía subtotal.
Reporte anatomopatológico de SG con ganglios linfáticos perigástricos e hiperplasia
folicular reactiva. En el seguimiento evolutivo a 24 meses se encuentra asintomática
en lo digestivo.
2. Discusión
Los tumores gastrointestinales subepiteliales se dividen en tres grandes grupos: neurogénicos
(schwannomas, neurofibromas), miogénicos (leiomiomas y leiomiosarcomas) y GIST. El
diagnóstico diferencial es importante, puesto que difieren en el pronóstico [3].
Los schwannomas son TM benignos de crecimiento lento, se originan en las células de
Schwann de los plexos Meissner y Auerbarch. Son infrecuentes, su ubicación más común
en el TGI es el estómago (a nivel de curvatura mayor y antro), seguido del colon y
el recto [3, 5].
Los SG son raros, una incidencia de 0,2 % de todos los tumores gástricos; 6,3 % de
los tumores gástricos mesenquimatosos; y 4 % de los tumores gástricos benignos [4]. Mayor prevalencia en mujeres, con una razón hombre:mujer de 1:3 y una edad promedio
al diagnóstico de 57 años [6,12].
La presentación clínica de los SG puede ser muy variable. La mayoría son asintomáticos
y su diagnóstico surge como un hallazgo. Los pacientes sintomáticos, frecuentemente,
experimentan dolor abdominal, seguido de hemorragia gastrointestinal alta. Con menor
frecuencia, presentan tumor abdominal palpable (3 %), anorexia (3 %) y dispepsia (1,8
%) [6].
La EDA, y las biopsias que de ella se obtienen, muestran bajo rendimiento [4]. Se evidencian como tumores subepiteliales sésiles, recubiertos de mucosa de aspecto
habitual con crecimiento exofítico [7]. El ultrasonido endoscópico identifica una lesión hipoecoica homogénea o heterogénea.
En muchas ocasiones, suele observarse un halo marginal que se localizan en la cuarta
capa y, en otros casos, en la tercera. La punción aspiración con aguja fina es el
método diagnóstico inicial, en el 85,2 %. Aunque, cuando el tejido obtenido es insuficiente
o inespecífico, la biopsia por punción con aguja gruesa presenta mejor rendimiento
[3, 8].
Otra herramienta diagnóstica utilizada es la TC contrastada que muestra tumoración
heterogénea hipervascular que realza al contraste, con áreas de necrosis, pero los
hallazgos radiológicos son inespecíficos y suelen describirlo como tumor del estroma
gastrointestinal [7, 13].
Histológicamente, los SG son encapsulados que contienen abundantes células fusiformes
con una agregación linfoide prominente, caracterizada por áreas de Antoni A y Antoni
B. Algunos autores demostraron que el diagnóstico solo puede confirmarse sobre la
base de IHQ en la que los SG muestran positividad para S-100, vimentina y proteína
gliofibrilar ácida, y negatividad para CD117 y SMA [3, 9, 10, 15].
En relación con el tratamiento, la cirugía es la única opción curativa para el SG,
y el tipo específico de procedimiento depende del tamaño y la localización de la lesión.
A su vez, tanto las técnicas convencionales como la laparoscópica han demostrado resultados
satisfactorios. La resección de ganglios linfáticos no es necesaria, ya que los SG
rara vez presentan diseminación linfática o transformación maligna. La opción endoscópica
no es viable, en la mayoría de los casos, ya que la lesión usualmente surge del plexo
de Auerbach y los crecimientos tienden a involucrar toda la capa muscular propia.
La tasa de recurrencia es muy rara por lo que no requiere vigilancia [7, 10, 14].
3. Conclusiones
Los schwannomas son TM benignos de crecimiento lento, se originan en las células de
Schwann de los nervios de los plexos Meiisner y Auebarch. Son poco frecuentes en el
TGI. Deben estar presentes en el diferencial de las lesiones subepiteliales diagnosticadas
por endoscopia.